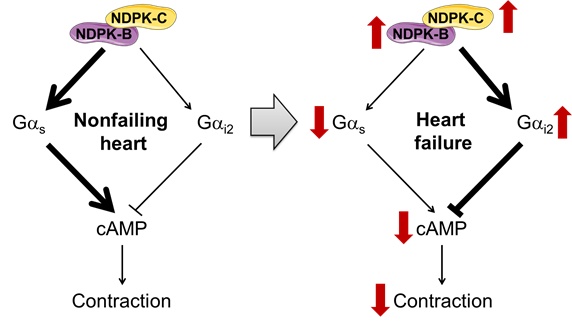
Heart failure (HF) remains a common cause of death and disability and is associated with altered signal transduction via b-adrenoceptors and G proteins, resulting in reduced adenylyl cyclase-mediated cAMP formation and contributing to contractile dysfunction. Nucleoside diphosphate kinases (NDPKs) can modulate G-protein activity and are enriched at the plasma membrane of end-stage HF patients. However, their relevance for HF pathophysiology is largely unknown, particularly for the NDPK‑C isoform.
Together with collaborators from various centers in Germany and abroad, Jordi Heijman published a study in the most recent edition of Circulation, showing for the first time a potential critical role for NDPK-C in the suppression of cAMP formation in HF patients. In particular, this study identified that NDPK-C is crucial for the interaction between NDPKs and G proteins, building complexes and scaffolding them at the plasma membrane. These interactions regulate cAMP levels and cardiomyocyte contractility. In HF patients, NDPK-C switched from predominantly Gas stimulation to activation of Gai. Our findings provide a potential mechanism for the detrimental decrease in cAMP and related dysfunction previously described in HF patients, and position NDPK‑C as a novel critical determinant of bAR/cAMP signaling that contributes to impaired cardiac function and remodeling in human HF.
Reference: Abu-Taha IH*, Heijman J*, Hippe HJ, Wolf NM, El-Armouche A, Nikolaev VO, Schäfer M, Würtz CM, Neef S, Voigt N, Baczkó I, Varró A, Müller M, Meder B, Katus HA, Spiger K, Vettel C, Lehmann LH, Backs J, Skolnik EY, Lutz S, Dobrev D#, Wieland T# (2016) Nucleoside Diphosphate Kinase-C Suppresses cAMP Formation in Human Heart Failure. Circulation, Published online on Dec 7, 2016. *equally contributing first authors, #co-senior and co-correspondence authors – [PubMed]
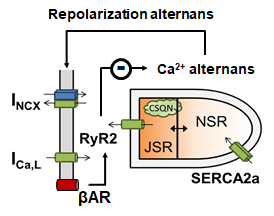 epolarization alternans has been implicated in ventricular arrhythmogenesis and sudden cardiac death, particularly in the infarct border zone (BZ). There exist conflicting data about the effect of sympathetic hyperinnervation on alternans formation and arrhythmogenesis in the BZ. However, detailed experimental characterization of the role of hyperinnervation in BZ arrhythmias is challenging due to the numerous distinct effects of post-infarction remodeling and the diversity of species-specific (sub)cellular mechanisms controlling calcium handling, alternans, and other pro-arrhythmic factors, all of which are modulated by sympathetic stimulation. In this manuscript, we characterized the interplay of BZ electrophysiological remodeling and sympathetic stimulation on alternans formation by fusing an existing state-of-the-art computational model of βAR stimulation in the canine ventricular cardiomyocyte with a model of post-infarction electrophysiological remodeling in the BZ. Our results indicate that βAR stimulation can indeed suppress alternans and we identified a critical role for the regulation of SR calcium release, both through activation of RyR2 channels and indirectly through regulation of SR calcium load, in the suppression of alternans.
epolarization alternans has been implicated in ventricular arrhythmogenesis and sudden cardiac death, particularly in the infarct border zone (BZ). There exist conflicting data about the effect of sympathetic hyperinnervation on alternans formation and arrhythmogenesis in the BZ. However, detailed experimental characterization of the role of hyperinnervation in BZ arrhythmias is challenging due to the numerous distinct effects of post-infarction remodeling and the diversity of species-specific (sub)cellular mechanisms controlling calcium handling, alternans, and other pro-arrhythmic factors, all of which are modulated by sympathetic stimulation. In this manuscript, we characterized the interplay of BZ electrophysiological remodeling and sympathetic stimulation on alternans formation by fusing an existing state-of-the-art computational model of βAR stimulation in the canine ventricular cardiomyocyte with a model of post-infarction electrophysiological remodeling in the BZ. Our results indicate that βAR stimulation can indeed suppress alternans and we identified a critical role for the regulation of SR calcium release, both through activation of RyR2 channels and indirectly through regulation of SR calcium load, in the suppression of alternans. 2017 is off to a good start for the lab with the publication of a podcast on cardiac computational modeling presented by Jordi Heijman, which was published on the website of the
2017 is off to a good start for the lab with the publication of a podcast on cardiac computational modeling presented by Jordi Heijman, which was published on the website of the 
 In response to the
In response to the  In response to the
In response to the